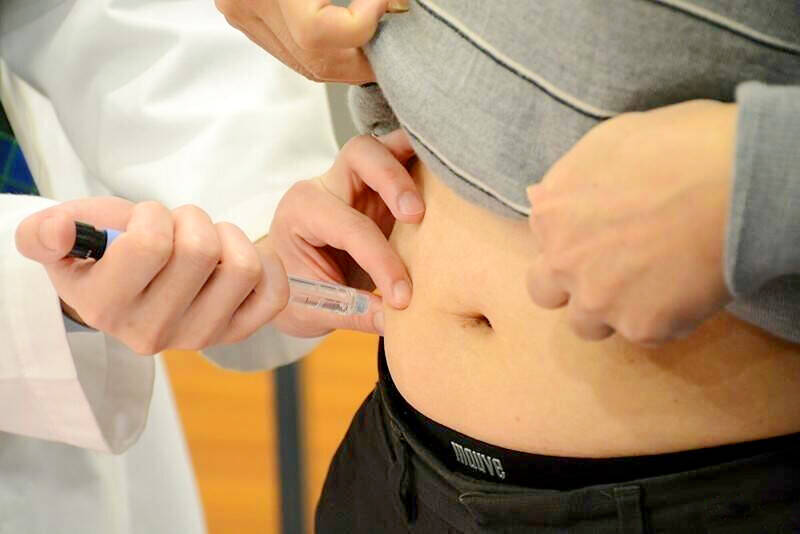
Weight loss drugs can cause illness, doctors say
The FDA issued a news release reminding the public to responsibly use the weight loss drugs, medication originally developed to treat diabetes by mimicking the GLP-1 hormone to lower blood sugar and induce a long-lasting feeling of fullness. The reported symptoms include gastrointestinal disorders, hypoglycemia (low blood sugar), nausea, vomiting and injection site discomfort, the FDA said. The agency said it has not observed any abnormalities in overall drug safety or new risks involving the GLP-1 drugs, but users should still remain mindful of side effects. “The ‘slimming injections’ are prescription drugs that must be evaluated and prescribed by a physician and dispensed by a pharmacist,” it said. “If an adverse reaction occurs, people should seek immediate medical attention,” it said, adding that it “will also continue to monitor local and international safety alerts on GLP-1 drugs, and will re-evaluate their safety, when necessary, to ensure public medication safety.”
Source: Taipei Times February 07, 2026 17:14 UTC