US FDA leaning toward approving Moderna half-dose booster–Bloomberg News
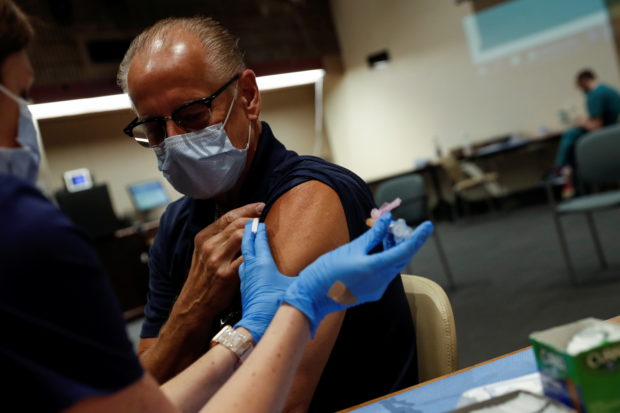
The U.S. Food and Drug Administration (FDA) is leaning toward authorizing half-dose booster shots of the Moderna Inc COVID-19 vaccine, Bloomberg News reported on Tuesday, citing people familiar with the matter. The FDA had been seeking information about the effectiveness of a full third dose of the Moderna vaccine, but is now ready to move forward and consider the half-dose booster Moderna has proposed, the report said. Moderna on Sept. 1 submitted its application to the U.S. Food and Drug Administration seeking authorization for a booster shot. The original Moderna vaccine contains 100-micrograms of mRNA in each shot. The company’s submission to regulators to authorize a half-dose booster would allow Moderna to produce more.
Source: Philippine Daily Inquirer September 29, 2021 02:26 UTC