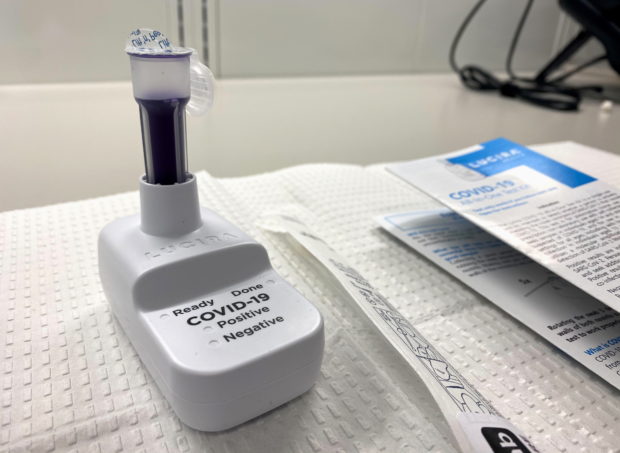
US FDA approves first COVID-19 test kit for home use
The U.S. Food and Drug Administration said on Tuesday it had approved the first COVID-19 self-testing kit for home use that provides results within 30 minutes. The single-use test, made by Lucira Health, has been given emergency use authorization for home use with self-collected nasal swab samples in individuals aged 14 and older who are suspected of COVID-19 by their health care provider, the FDA said. ADVERTISEMENT“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” FDA Commissioner Stephen Hahn said. “We look forward to proactively working with test developers to support the availability of more at-home test options,” said Jeff Shuren, the director of the FDA’s Center for Devices and Radiological Health. For more information on COVID-19, call the DOH Hotline: (02) 86517800 local 1149/1150.
Source: Philippine Daily Inquirer November 19, 2020 23:03 UTC







