Here's what we know about Pfizer's vaccine -- including who could get it first
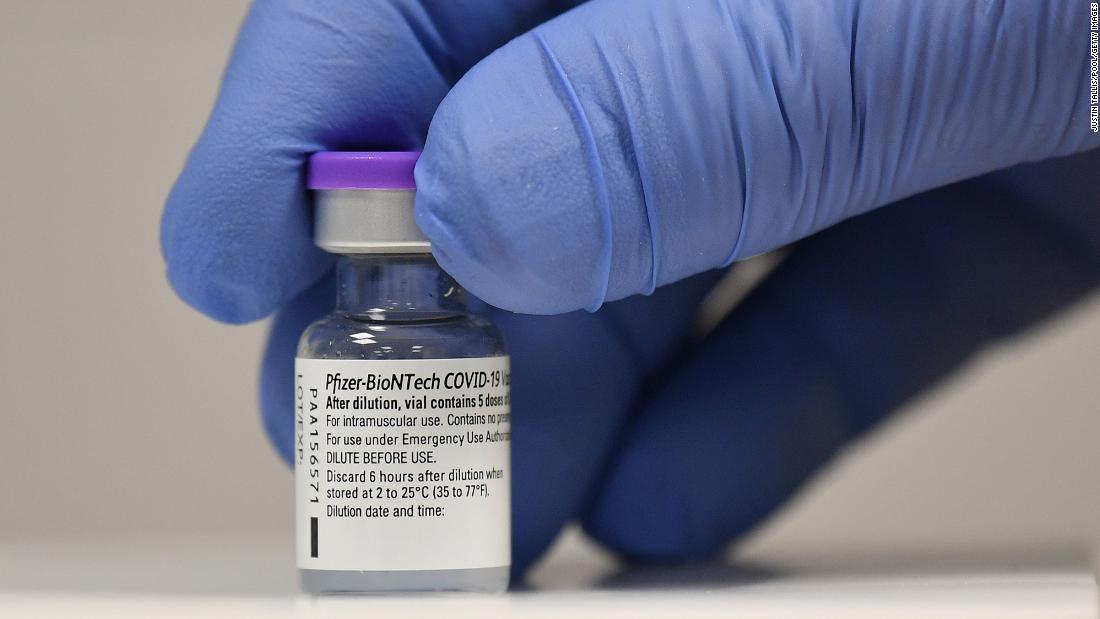
(CNN) The US Food and Drug Administration has issued emergency use authorization (EUA) for the Covid-19 vaccine developed by Pfizer and BioNTech. Here's what we know about the Pfizer/BioNTech Covid-19 vaccine. Azar said earlier this month that 6.4 million doses of Pfizer vaccine would be allocated for shipment the first week. But Pfizer's vaccine needs to be stored at incredibly cold temperatures , making the logistics of delivery even more complicated. It also remains unclear how safe the vaccine is for other groups, like pregnant women and children under 16.
Source: CNN December 11, 2020 21:26 UTC