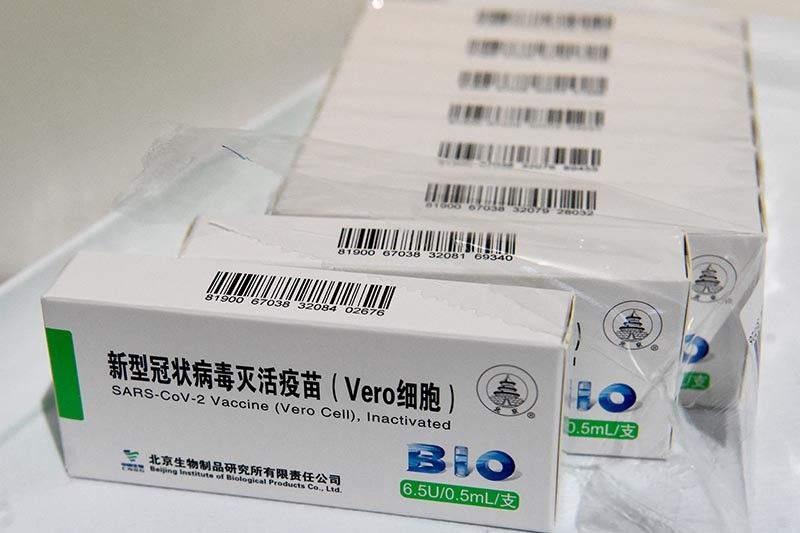
DOH to seek emergency use authorization for Sinopharm COVID-19 vaccine
MANILA, Philippines — The Department of Health will file an application seeking an emergency use authorization for the COVID-19 vaccine developed by Chinese state-owned company Sinopharm, its chief said Monday. The FDA has issued EUAs to vaccines made by Pfizer and BioNTech, AstraZeneca, Sinovac, Gamaleya Research Institute, Johnson & Johnson, Bharat Biotech and Moderna. The COVID-19 vaccine developed by Sinopharm became the first Chinese jab to receive the WHO’s green light. The United Nations health agency recommended the two-dose vaccine for adults 18 years and older. The WHO also granted emergency use listing to the vaccines being made by Pfizer-BioNTech, Moderna, Johnson and Johnson, and AstraZeneca.
Source: Philippine Star May 10, 2021 01:52 UTC