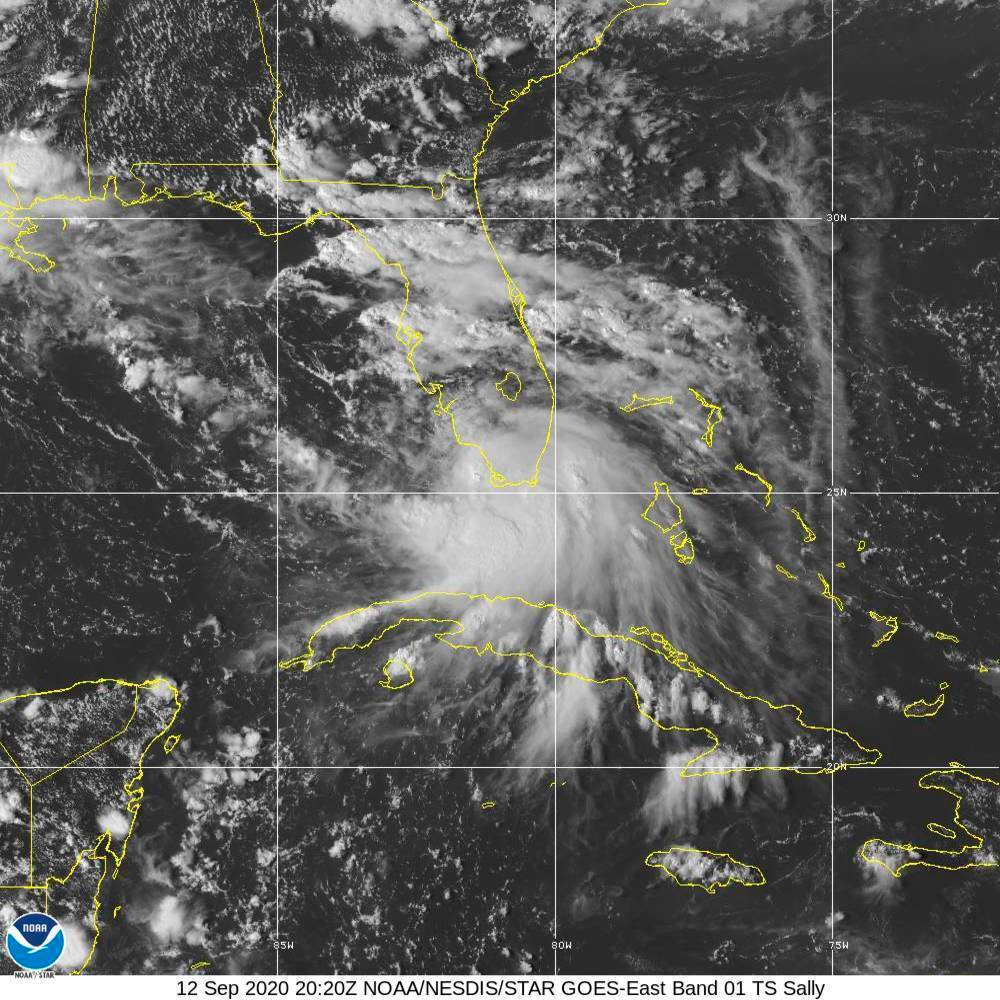
Covid-19 vaccine: How an EUA can impact the timeline
When the health emergency is over, "then any EUA(s) issued based on that declaration will no longer remain in effect," according to the FDA. After about two and a half months with EUA, the emergency authorization was revokedMany experts see granting an EUA to a vaccine against Covid-19 as problematic. Without a doubt, the pace of medical innovation has moved faster than ever before, and human vaccine trials began just 67 days after the virus was first identified. If the vaccine were administered by a public health professional, 55% of respondents say they would take it. Moncef Slaoui, chief adviser to Operation Warp Speed, the federal government's Covid-19 vaccine program, said that "it would be unethical" to not move quickly to put out a Covid-19 vaccine if it is proven to work.
Source: CNN September 13, 2020 11:37 UTC